Is Glucose a reducing sugar?
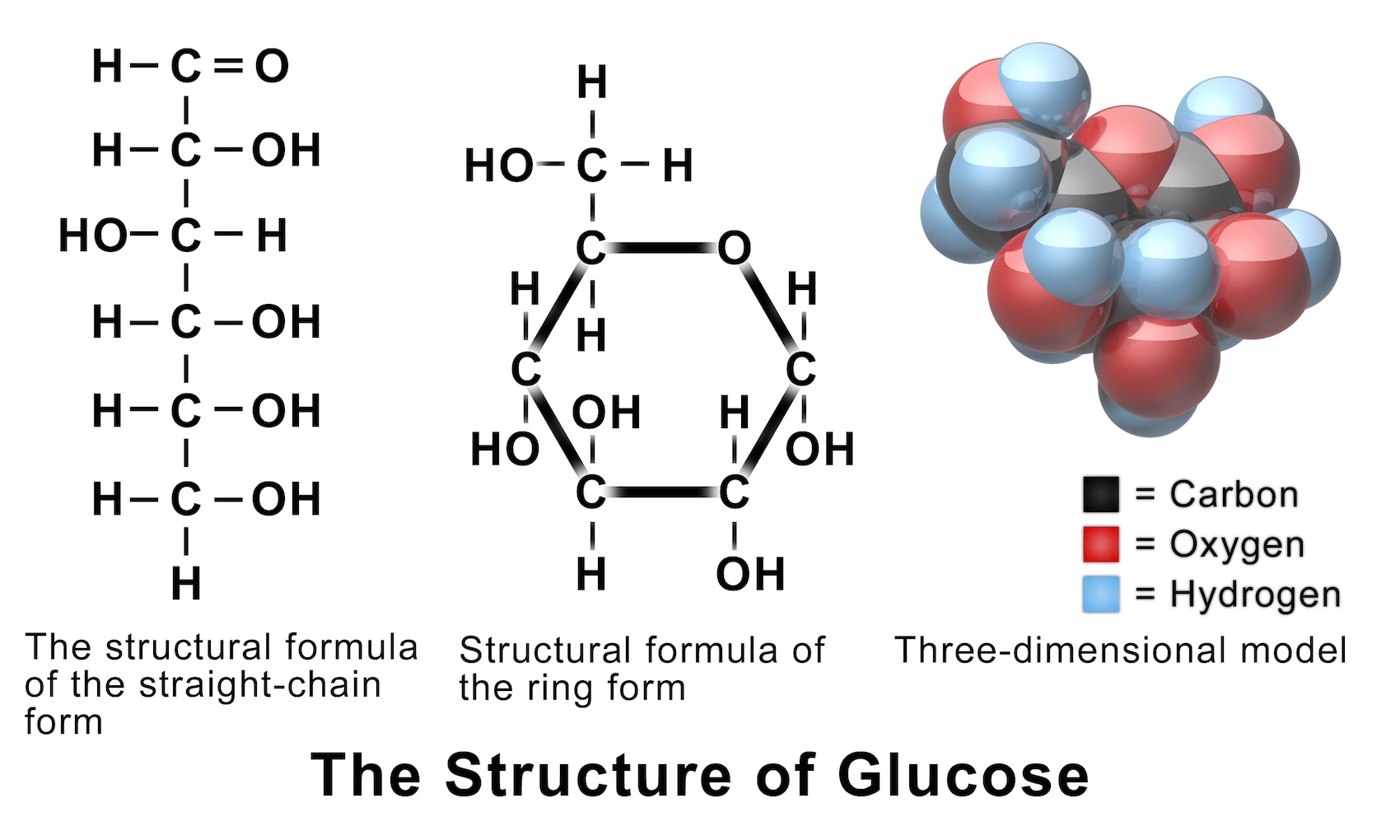
glucose, one of a group of carbohydrates known as simple sugars ( monosaccharides ). Glucose (from Greek glykys; "sweet") has the molecular formula C 6 H 12 O 6. It is found in fruits and honey and is the major free sugar circulating in the blood of higher animals. It is the source of energy in cell function, and the regulation of its.
PPT Solutions & Solubility PowerPoint Presentation, free download
Note that they are all named using the suffix -ose, which means sugar. Carbohydrates are often named "somethingose". Figure 3.3.1 3.3. 1 These monosaccharides respect the ratio 1:2:1 mentioned above: glucose (C 6 H 12 O 6 ), fructose (C 6 H 12 O 6 ), galactose (C 6 H 12 O 6 ), ribose (C 5 H 10 O 5 ), deoxyribose (C 5 H 10 O 4, this one is.
LGlucose Wikipedia
Only today, enjoy all categories up to 90% off your purchase. Hurry & shop mow. Awesome prices & high quality here on Temu. New users enjoy free shipping & free return.

Glycolysis and Gluconeogenesis The Journey to Pyruvate and
List and distinguish the major organic molecules (sugars and starches; amino acids and proteins, nucleotides and nucleic acids; fatty acids, phospholipids, trigylcerides, and cholesterol) and explain how polymers provide for increasingly complex molecules. Distinguish between covalent and ionic chemical bonds.

SolubilityPolarity YouTube
4.4 Solubility. 4.3 Boiling Points. 4.5 Chromatography. An understanding of bond dipoles and the various types of noncovalent intermolecular forces allows us to explain, on a molecular level, many observable physical properties of organic compounds. In this section, we will concentrate on solubility, melting point, and boiling point.

Is Glucose Polar or Nonpolar (C6H12O6) YouTube
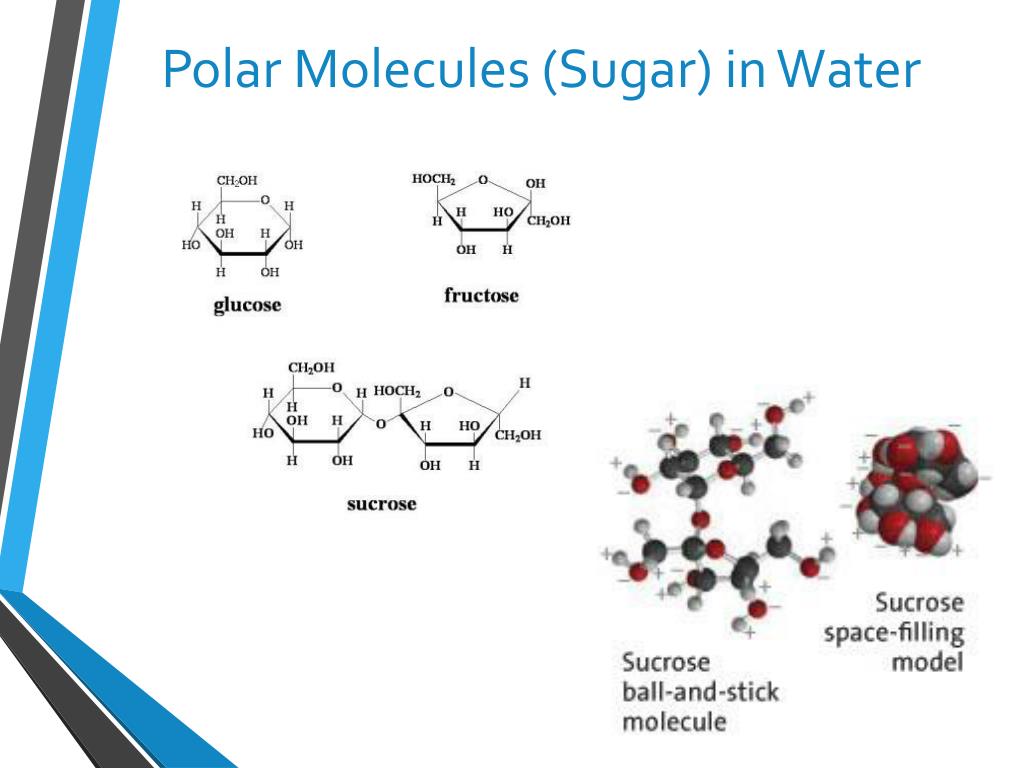
Is glucose a polar molecule? Q: Is glucose a polar molecule? Is glucose a polar molecule? Flexi Says: The sugar glucose is a covalent compound. When sugar dissolves in water, it forms individual glucose molecules (C 6 H 12 O 6 ). You can see how this happens in the Figure here.

PPT Atoms and Molecules PowerPoint Presentation, free download ID
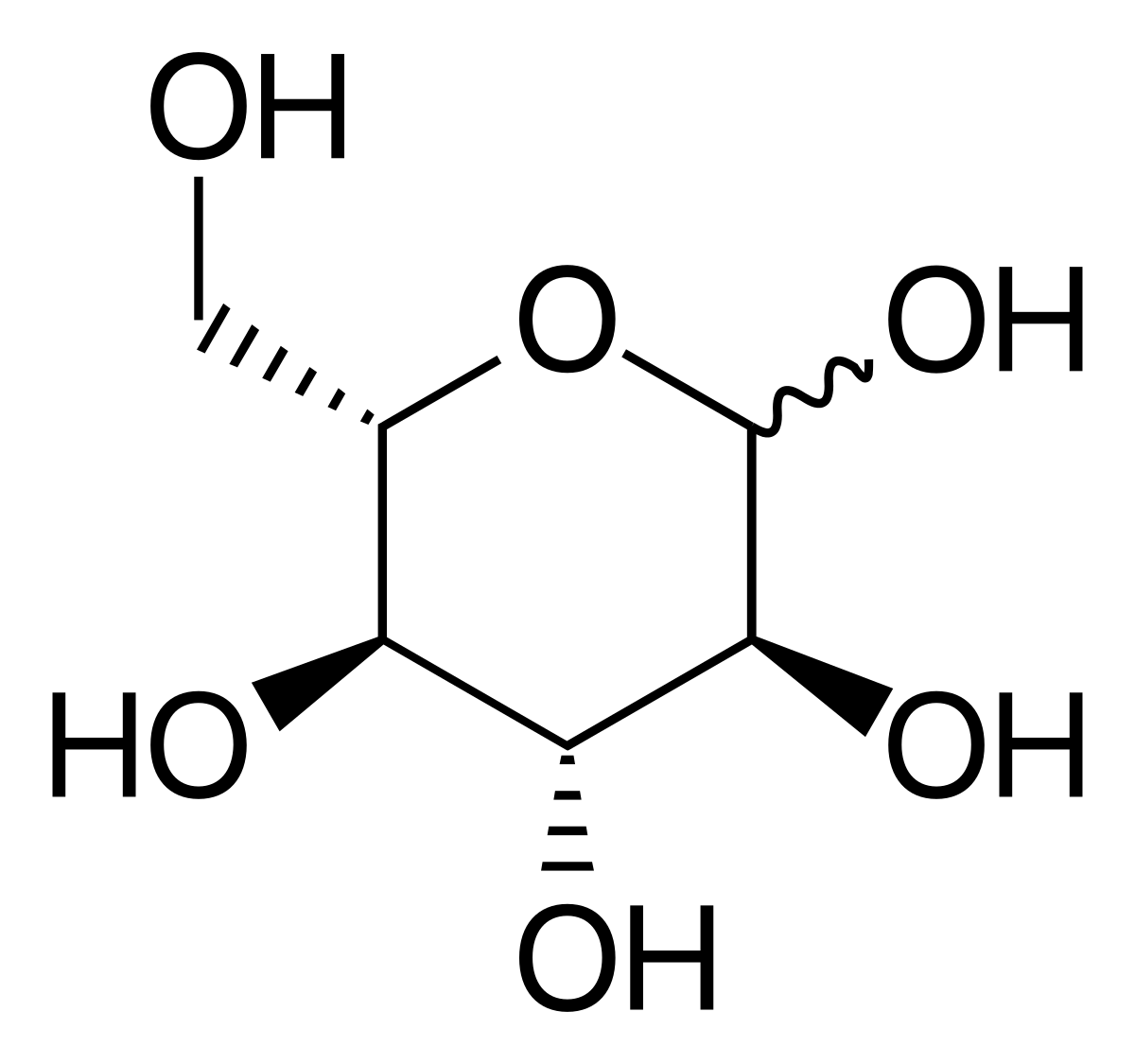
Glucose (C6H12O6) is a polar molecule. Glucose is a six-carbon compound. It is a polyhydroxy aldehyde i.e.; it consists of five hydroxyls (OH) functional groups and an aldehyde (CHO) group at carbon number 1.

PPT Molecular Structure & Intermolecular Forces PowerPoint
1. Although weak, multiple hydrogen bonds are important in stabilizing the three-dimensional shape of many biological molecules. (Shape determines function "functional conformation") 2. Covalent bonds are usually very stable. -cells use protein catalysts called enzymes to "break" covalent bonds e.g. hydrolysi.

Molecular Formula of Glucose BrodericksrGould
Glucose is a key energy source for most living cells. Due to its polar nature and large size, glucose molecules cannot traverse the lipid membrane of the cell by simple diffusion. Instead, the entry of glucose molecules into the cells is effected by a large family of structurally related transport proteins known as glucose transporters.
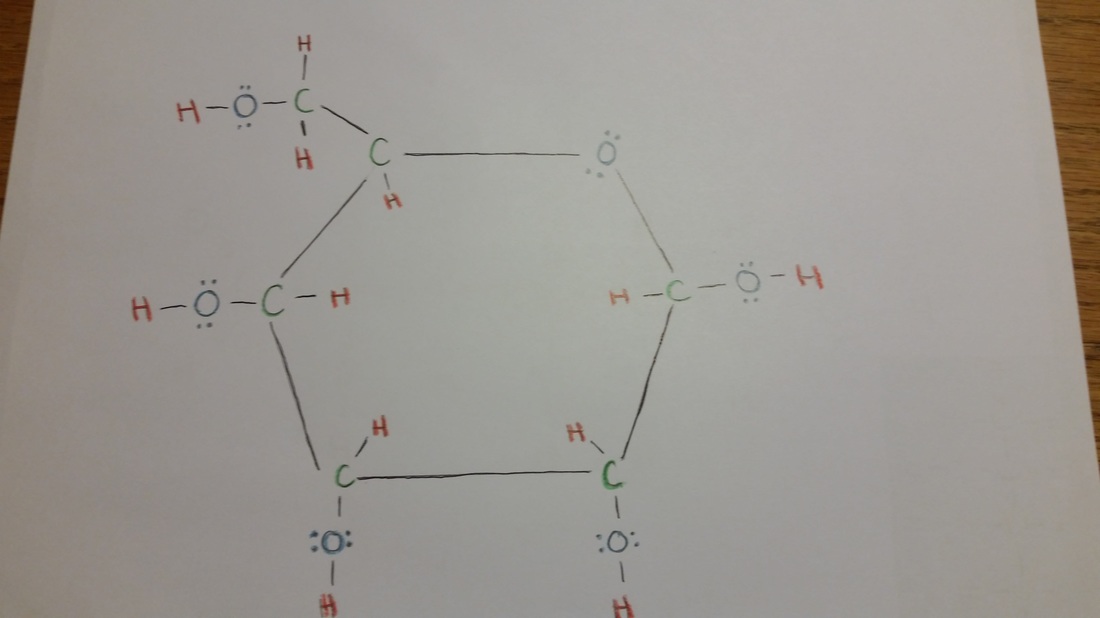
Lewis Structure Linear and Cyclic Glucose
Trial Australia's smallest, thinnest continuous glucose monitor today for $15*. Upgrade from blood glucose monitors. One app. No transmitter. No calibration required.

Sugar Polar or Nonpolar YouTube
Chemical and physical properties Glucose forms white or colorless solids that are highly soluble in water and acetic acid but poorly soluble in methanol and ethanol.

Glucose Chemical Formula Everything You Need to Know science issue
Henry Agnew (UC Davis) 5.10: Electronegativity and Bond Polarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken if energy is added to a molecule.
[Solved] Why monosaccharides are more polar than disaccharides
Glucose is sweet because it contains OH groups with a certain orientation that interacts with the taste receptor for sweetness in our tongues. This is the same reason that fructose is sweet. 3 comments

Glucose Metabolism Diagram glucose as a hormone receptormediated
Is glucose polar? Question: Is glucose polar? Polar Molecules When two molecules are nonmetals, the bond between them can be classified as polar or nonpolar. If the electrons are shared.

Glucose (dextrose, Dglucose) molecule. Сyclic and acyclic forms
There are two basic types of covalent bonds: polar and nonpolar. In a polar covalent bond , the electrons are unequally shared by the atoms and spend more time close to one atom than the other. Because of the unequal distribution of electrons between the atoms of different elements, slightly positive (δ+) and slightly negative (δ-) charges develop in different parts of the molecule.

Glucose (polar) Chemical Formula
Answer link "Sugar is a highly polar molecule." Glucose, C_6H_12O_6, has 4 secondary hydroxyl groups, and 1 (exocyclic) primary hydroxyl group. Sugar is a highly polar molecule that has substantial water solubility.